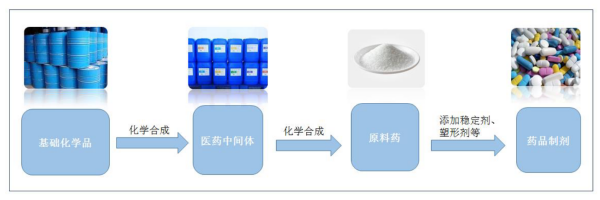
Pharmaceutical intermediates are intermediate substances in the synthesis process of apis, which belong to pharmaceutical fine chemicals, and do not require drug production licenses. According to the impact on the quality of the final apis, they can be divided into non-GMP intermediates and GMP intermediates.
The pharmaceutical intermediates industry refers to those chemical enterprises that produce and process organic/inorganic intermediates or apis used in the manufacture of finished drugs for pharmaceutical enterprises by chemical synthetic or biosynthetic methods in accordance with strict quality standards.
(1) The pharmaceutical intermediates sub-industry can be subdivided into CRO and CMO industries.
CMO refers to the contract manufacturing enterprise, which means that the pharmaceutical company outsources the production process to a partner.
The pharmaceutical CMO industry needs to purchase basic chemical raw materials and classify them into special pharmaceutical raw materials, and then reprocess them into starting materials, cGMP intermediates, apis and preparations.
At present, major multinational pharmaceutical companies tend to establish long-term strategic partnerships with a small number of core suppliers, and the survival of companies in the industry is basically clear through their partners.
A CRO is a contract research organization that a pharmaceutical company contracts out to a partner.
At present, the industry mainly adopts customized production, customized research and development, and pharmaceutical contract research and sales as the main cooperation methods, no matter which way, regardless of whether pharmaceutical intermediates are innovative products
The evaluation of the core competitiveness of enterprises is still based on research and development technology, which is reflected as the downstream customers or partners of the company.
(2) From the classification of business models, intermediate enterprises can be divided into general mode and custom mode.
In general, intermediate production enterprises adopt a general model, facing more customers are generic drug manufacturers, and large intermediate manufacturers with strong research and development capabilities adopt a customized model, customer-oriented innovative drug enterprises, customized model can effectively enhance the viscosity between customers.
Under the general product model, enterprises identify the general needs of mass customers according to the results of market research, and carry out research and development, production, sales and other specific business activities as a starting point.
In the pharmaceutical industry, the generic product model is mainly applicable to the research and development, production and sales of pharmaceutical intermediates, apis and preparations required for generic drugs.
In the customized mode, the customized customer provides confidential information to the enterprise after signing a confidentiality agreement with the enterprise, and defines the customization needs. Enterprises to customize the customized needs of customers as a starting point to carry out research and development, production, sales and other specific business activities.
In the pharmaceutical industry, the customized model is mainly applicable to the research and development, production and sales of pharmaceutical intermediates, apis and preparations required for innovative drugs.
Due to the large market space of China’s pharmaceutical demand, coupled with obvious technological progress, not only the rapid development of pharmaceutical intermediates, but also led to the domestic market competition is also extremely fierce
However, most of China’s local CRO companies have relatively single business, and their competitiveness is weak compared with international giants, and the gap is still obvious. But because of this, China’s CRO industry is also facing great potential for development. For example, Xinxiang (Shenzhen) Biology is a CRO company engaged in pre-clinical research, innovative research and development of new drugs, efficient chemical synthesis, compound screening, process and quality standards research pharmacology and toxicology experiments and other business content, can also provide customers with the whole process of research and development technical services.
In the face of this good opportunity, domestic pharmaceutical machinery enterprises also need to base on their own development positioning, through creating a scientific strategy, continuous innovation, break the dilemma of product homogeneity, and walk out of a road that meets market trends and meets customer needs in order to win a greater market share
About us
Changzhou Zhichao Medical Technology Co., LTD., founded in September 2011, is headquartered in Changzhou Hang Seng Science and Technology Industrial Park. Today, the company’s R&D center is engaged in the process research and development, production, optimization, custom synthesis and technology transfer of API or pharmaceutical intermediates, plant extracts, compounds from laboratory scale to commercial scaleup production, to meet the growing requirements of customers.
Zhichao Chemical attaches great importance to research and development and technology investment, and has established long-term strategic cooperation with domestic and foreign science academies, and has a branch in India. The team has developed and cooperated with related preparation projects, and difficult, small market volume, high value-added products, and in strict accordance with the international GMP standard management, products are exported to Europe, America, India, Southeast Asia, the Middle East and South America and other markets. At the same time, the company pays attention to the protection of intellectual property rights, constantly invents and innovates research and development of production processes, circumvent patent and intellectual property problems in the regulatory market, perfect impurity research, and provide customers with a full set of CMC or DMF registration documents.
The company adheres to the business philosophy of honesty, quality service, mutual benefit and win-win, the company culture of enthusiasm, openness and sharing, and embraces the guidance and common promotion of domestic and foreign counterparts.
